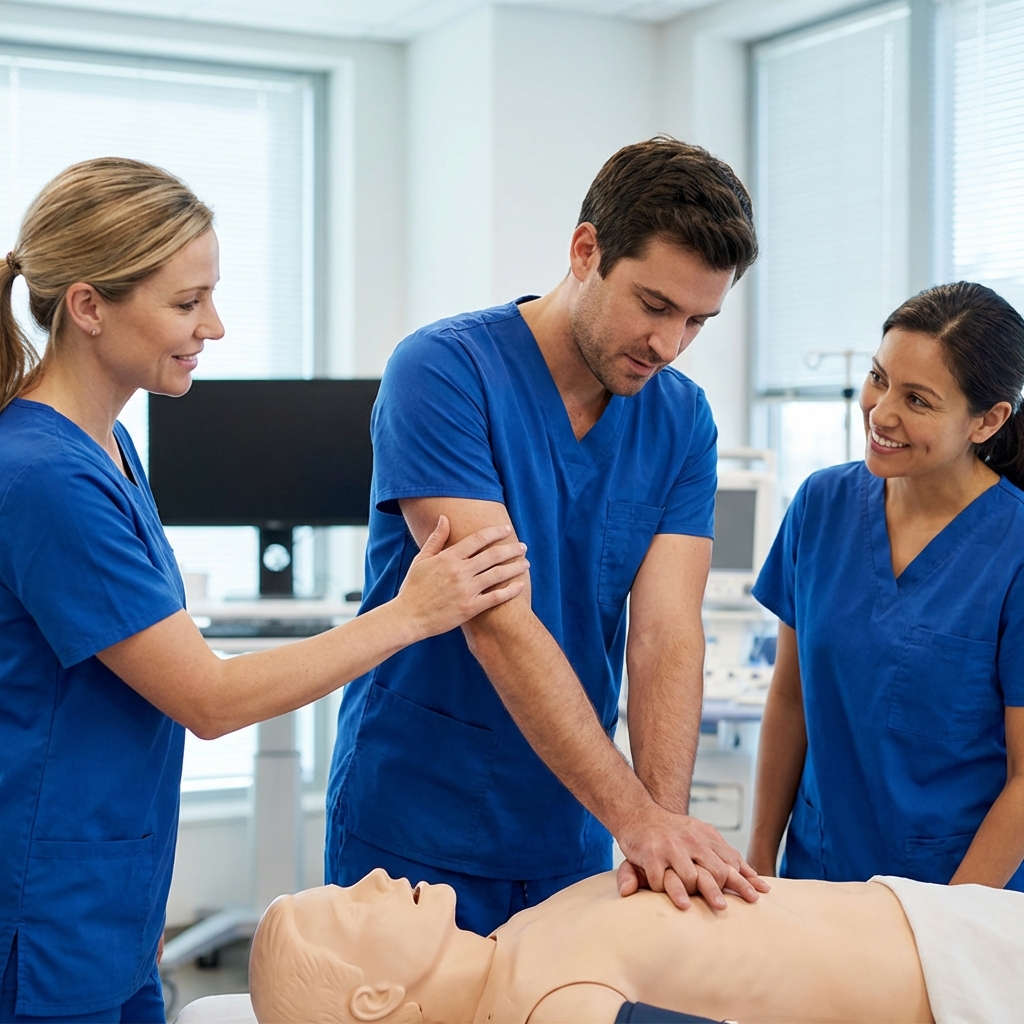
Enhanced Real-Time Feedback for High-Quality Chest Compressions
Among the AHA 2025 guideline changes, expect a stronger emphasis on real-time audiovisual feedback to drive consistent, high-quality compressions at the bedside. This aligns with resuscitation science updates showing that teams who monitor and correct rate, depth, recoil, and interruptions in the moment achieve higher ROSC and survival. Build feedback devices into your ACLS and PALS algorithms—treat them like any other critical monitor alongside rhythm checks and capnography.
Focus your practice around concrete CPR performance metrics. Adults: deliver 100–120 compressions per minute at a depth of about 2–2.4 inches (5–6 cm), allow full recoil, and minimize pauses; if an advanced airway is placed, ventilate about 10/min. Pediatrics: target 1/3 anterior–posterior chest depth (about 1.5 inches for infants, 2 inches for children) at 100–120/min. Use end-tidal CO2 to gauge perfusion during compressions (values ≥10 mm Hg suggest adequate quality and rising ETCO2 can herald ROSC); when an arterial line is present in children, aim for diastolic blood pressure ≥25–30 mm Hg, consistent with prior pediatric guidance.
Practical bedside steps to operationalize feedback within advanced cardiac life support protocols and pediatric advanced life support changes:
- Pre-brief: confirm defibrillator or puck-based accelerometer feedback is connected and calibrated; turn on a metronome.
- Assign a CPR coach to watch the feedback display and call out corrections in real time.
- Rotate compressors every 2 minutes or sooner if depth or rate trends down on the device.
- Optimize surface with a backboard; adjust hand position and body mechanics when the device flags shallow depth or incomplete recoil.
- Maximize compression fraction; many teams target >80% as a quality goal while keeping rhythm and shock pauses under 10 seconds.
- Track ETCO2 and use it with device data to guide adjustments; a sudden ETCO2 rise can prompt a rapid pulse check.
- Post-event, export feedback reports to fuel debriefs and align with post-cardiac arrest care standards and QI dashboards.
Example: During an ED cardiac arrest, the accelerometer shows depth drifting to 1.7 inches and recoil alerts appear. The CPR coach cues “deeper, full release,” adds a backboard, and turns on a 110/min metronome; ETCO2 rises from 8 to 16 mm Hg, and the team shortens pre-shock pauses to under 8 seconds—ROSC follows after the next cycle.
Safety Training Seminars integrates device-enabled manikins and coaching into blended BLS, ACLS, and PALS courses across 100+ California locations, so you can apply these updates under realistic conditions. If you practice sedation or office-based anesthesia, review your ACLS and PALS certification requirements and ensure your team trains with feedback-equipped gear to meet evolving standards.
Updated Medication Dosages in Pediatric Advanced Life Support
Among the AHA 2025 guideline changes, pediatric medication guidance is expected to emphasize precision, standardization, and error reduction at the bedside. The focus aligns with resuscitation science updates that seek to limit calculation time, reduce variability in concentrations, and support CPR performance metrics by minimizing pauses for drug preparation. For California teams, the practical takeaway is to verify dose bands, max doses, and dilution conventions in your local PALS algorithms and code carts as soon as updates are released.
Translate new tables directly into weight-based workflows. Build pre-calculated dose charts in 5 kg increments, integrate color-coded length-based tapes, and standardize concentrations in Pyxis/code boxes to match smart-pump libraries. Ensure IO access is treated as equivalent to IV for delivery of resuscitation meds, and map drug timing intervals (for repeat dosing) onto your defibrillation and rhythm-check cycles to avoid unnecessary interruptions.
Priority medications to reconcile in your bedside algorithms include:
- Epinephrine (cardiac arrest, bradycardia): verify 0.01 mg/kg of 0.1 mg/mL (1:10,000) per dose, max single dose, and 3–5 minute interval alignment with rhythm checks; pre-calculate volumes in mL.
- Amiodarone or lidocaine (shockable arrest): confirm mg/kg bolus, cumulative dose caps, and infusion transition post-ROSC for refractory VF/VT.
- Adenosine (stable SVT): validate first/second-dose mg/kg calculations, 6 mg/12 mg maximums, and rapid IV push with proximal access and flush.
- Magnesium (torsades, suspected hypomagnesemia): confirm mg/kg range, infusion time, and maximum dose.
- Calcium (hyperkalemia, hypocalcemia, CCB overdose): specify when indicated and which salt (chloride vs gluconate) is stocked, with mg/kg and rate.
- Dextrose (hypoglycemia): standardize weight-based grams/kg and the preferred concentration (D10 vs D25) by age, with mL/kg to reduce calculation errors.
- Naloxone (respiratory depression): set initial mg/kg and titration strategy to avoid abrupt withdrawal.
- Atropine (bradycardia with AV block): verify mg/kg, minimum dose, and repeat limits.
Concrete examples to speed bedside delivery: for a 15 kg child in pulseless arrest, epinephrine 0.01 mg/kg equals 0.15 mg or 1.5 mL of 0.1 mg/mL. Amiodarone 5 mg/kg equals 75 mg; prepare the infusion with smart-pump guardrails. For a 30 kg adolescent with SVT, adenosine 0.1 mg/kg is 3 mg (first dose), followed by 0.2 mg/kg = 6 mg (second dose), if needed.
Close the loop by updating order sets, smart-pump libraries, and bedside cards so pediatric advanced life support changes stay consistent with adult advanced cardiac life support protocols and pediatric post-cardiac arrest care standards. Safety Training Seminars integrates the latest AHA 2025 guideline changes into scenario-based PALS courses across 100+ California locations, with blended learning and group options to standardize dosing practices. Teams can also refresh foundational skills through updated BLS training courses to support high-quality compressions while medications are prepared and delivered.
Streamlined Targeted Temperature Management for Post-Arrest Care
Among the most impactful AHA 2025 guideline changes is a streamlined approach to targeted temperature management (TTM) in post-cardiac arrest care. The emphasis shifts from routine induction of hypothermia to active prevention of fever and sustained controlled normothermia. For bedside teams, this simplifies decision-making: prioritize continuous core temperature monitoring and intervene to keep temperature in the normal range while integrating hemodynamic, respiratory, and neurologic care.
At the bedside, translate these resuscitation science updates into a clear, repeatable algorithm:
- Place a continuous core temperature probe (esophageal, bladder, or intravascular) as soon as ROSC is secured.
- Initiate measures to prevent fever (commonly ≤37.5°C) for 24–72 hours: antipyretics, surface cooling pads, or intravascular devices to maintain normothermia.
- Use sedation and shiver control bundles (e.g., adequate analgesia, buspirone, magnesium; consider meperidine or short-term neuromuscular blockade as rescue) to avoid temperature spikes.
- If hypothermia is induced or present on arrival, rewarm slowly (about 0.25–0.5°C/hour) and avoid overshoot fevers.
- Defer neurologic prognostication until at least 72 hours after return to normothermia, with continuous EEG when feasible to detect subclinical seizures.
These updates align advanced cardiac life support protocols with the latest evidence (e.g., no routine benefit to deep hypothermia) while preserving clinician judgment for select cases. For example, a comatose adult after out-of-hospital VF arrest should have temperature controlled to normothermia with surface pads within the first hour, acetaminophen scheduled, and a shiver protocol initiated; routine targeting of 33°C is no longer the default. Conversely, a patient arriving spontaneously at 34°C should be rewarmed cautiously to the normal range without rapid active warming that risks rebound hyperthermia.
Expect pediatric advanced life support changes to mirror this direction: prioritize fever prevention and controlled normothermia in children after ROSC, while recognizing separate neonatal pathways (NRP) for hypoxic-ischemic encephalopathy. For all ages, integrate TTM with post-cardiac arrest care standards—optimize oxygenation and ventilation (avoid hyperoxia and hypocapnia), maintain MAP targets, treat seizures promptly, and standardize sedation-analgesia.

To drive quality, add CPR performance metrics that extend into the post-arrest phase: time to temperature control, percent time in target range, fever-burden hours, and adherence to shiver protocols. California hospitals updating order sets and education around AHA 2025 guideline changes can leverage Safety Training Seminars for ACLS, PALS, and NRP courses that incorporate the new temperature-management algorithms. With blended learning and skills sessions across 100+ California locations, teams can rapidly align bedside practice with the latest post-cardiac arrest care standards.
Revised Airway Management Protocols for Adult Cardiac Arrest
The AHA 2025 guideline changes continue to reinforce a compressions-first approach while refining how teams select and execute airway strategies during adult cardiac arrest. The emphasis is on minimizing interruptions, selecting the airway that can be placed most rapidly with the highest likelihood of success, and using continuous waveform capnography for confirmation and quality feedback. Video laryngoscopy is favored when available and when it improves first-pass success, and supraglottic airways (SGAs) remain appropriate when intubation would delay compressions.
At the bedside, start with two-rescuer bag-mask ventilation (BMV) using an OPA/NPA and a tight mask seal; once an advanced airway is placed, ventilate at 1 breath every 6 seconds (10/min) with continuous compressions, avoiding hyperventilation. Limit intubation attempts and keep any pauses under 10 seconds, maintaining a chest compression fraction above 80%. Use end-tidal CO2 to confirm placement and guide performance; a sudden ETCO2 rise may signal ROSC, while persistently low values can prompt checks of compression depth/rate and ventilation.
Key actions to implement now based on these resuscitation science updates and advanced cardiac life support protocols:
- Choose the airway that minimizes no-flow time: early SGA when intubation will be prolonged; endotracheal tube when an experienced operator and equipment are immediately ready.
- Prefer video laryngoscopy and consider a bougie to improve first-pass success and shorten attempts.
- Confirm every airway with continuous waveform capnography and clinical assessment; do not rely on auscultation alone.
- Ventilate with 100% oxygen during arrest, then after ROSC titrate to SpO2 92–98% and target normocapnia (PaCO2 35–45 mm Hg) as part of post-cardiac arrest care standards.
- Track CPR performance metrics in debriefings: peri-intubation pause time, ventilation rate, chest compression fraction, and ETCO2 trends.
- Avoid routine cricoid pressure if it impairs ventilation or visualization, and suction aggressively when airway contamination is present.
For example, in a witnessed VF arrest with heavy emesis, place an SGA rapidly while compressions continue, confirm with capnography, and defer endotracheal intubation until an expert laryngoscopist and video scope are ready to keep the pause under 10 seconds. After ROSC, switch from 100% oxygen to a titrated target and maintain normocapnia while continuing ETCO2 monitoring. Note that pediatric advanced life support changes differ in compression-to-ventilation ratios and equipment sizing; avoid directly transplanting adult steps into pediatric algorithms.
Safety Training Seminars integrates these AHA 2025 guideline changes into ACLS refreshers and skills sessions across California, with blended learning and hands-on practice using SGAs, video laryngoscopes, and capnography. With over 100 locations and a low price guarantee, clinicians can rapidly update competencies while meeting compliance requirements. Group scheduling and tailored scenarios also support hospital teams working to improve airway performance metrics and outcomes.
Refined Algorithm for Tachycardia with a Pulse Management
Among the AHA 2025 guideline changes, the tachycardia-with-a-pulse pathway is refined to reduce delays between recognition of instability and synchronized cardioversion, while preserving familiar advanced cardiac life support protocols. Clinicians should rapidly classify patients as unstable if hypotension, altered mental status, signs of shock, ischemic chest discomfort, or acute heart failure are present. Place defibrillation pads early, obtain a 12‑lead ECG if it won’t delay therapy, and prepare sedation while ensuring immediate access to electricity.
For unstable rhythms, perform synchronized cardioversion without delay. Typical energy selections remain: narrow regular 50–100 J; narrow irregular (e.g., atrial fibrillation) 120–200 J biphasic; wide regular (monomorphic VT) 100 J. Avoid synchronized mode for polymorphic or irregular wide-complex tachycardias—use defibrillation doses. Provide analgesia/sedation as feasible, but do not postpone shock in a deteriorating patient.
Stable narrow-complex regular tachycardia still prioritizes vagal maneuvers (modified Valsalva) followed by adenosine 6 mg rapid IV push, then 12 mg if needed. If the rhythm is irregular narrow-complex (AF/flutter), consider rate control with a beta blocker (e.g., metoprolol) or a nondihydropyridine calcium channel blocker (diltiazem/verapamil) in hemodynamically stable patients. Avoid AV nodal blockers in pre-excited AF (WPW features); favor procainamide or synchronized cardioversion.
For stable wide-complex tachycardia, a structured, drug-first approach remains appropriate when monomorphic and well-tolerated:
- Procainamide: 20–50 mg/min until arrhythmia suppresses, hypotension, QRS widens >50%, or max dose reached; maintain 1–4 mg/min (avoid in heart failure/prolonged QT).
- Amiodarone: 150 mg IV over 10 minutes; repeat as needed, then infusion.
- Sotalol: 100 mg (1.5 mg/kg) IV over 5 minutes; avoid with prolonged QT.
Administer magnesium sulfate (1–2 g IV) for torsades de pointes. Seek expert consultation early and reassess frequently for signs of instability.
Operational refinements emphasized in recent resuscitation science updates include earlier comprehensive rhythm documentation, bedside ultrasound to assess perfusion when uncertainty exists, and readiness to pivot to the cardiac arrest algorithm if pulses are lost. If decompensation occurs, apply high-quality CPR performance metrics (rate 100–120/min, full recoil, minimal pauses) and follow post-cardiac arrest care standards after ROSC. Parallel pediatric advanced life support changes preserve this decision logic but with different dosing: adenosine 0.1 mg/kg (max 6 mg) then 0.2 mg/kg (max 12 mg), and synchronized cardioversion starting at 0.5–1 J/kg, then 2 J/kg.
To put the AHA 2025 guideline changes into practice, California teams can train with Safety Training Seminars across more than 100 locations or via blended learning. Their ACLS and PALS courses align with current evidence, include rhythm interpretation drills for complex tachyarrhythmias, and support group bookings with a low price guarantee—ideal for nurses, dentists, and EMS personnel maintaining AHA-mandated certifications.

Integration of Advanced Hemodynamic Monitoring During Resuscitation
One of the most consequential AHA 2025 guideline changes is a stronger push to individualize CPR using real‑time physiologic data. These resuscitation science updates move bedside practice beyond fixed routines to titrated care driven by ETCO2, arterial diastolic pressure, and CPR performance metrics. For California clinicians, integrating these signals into ACLS and PALS decision trees will tighten feedback loops and can improve perfusion during low‑flow states.
Waveform capnography remains foundational. For intubated patients, maintain continuous ETCO2 monitoring; values persistently below about 10 mmHg suggest inadequate chest compression quality, excessive ventilation, or severe low-flow. A sudden, sustained ETCO2 rise often heralds ROSC and should trigger a rhythm check at the next planned pause. Reconfirm tube placement and reduce ventilation to about 10 breaths/min in adults to avoid hyperventilation.
When an arterial line is present (ICU, OR, some ED arrests), use diastolic arterial pressure to guide compressions and vasopressors. Adult targets commonly cited in prior guidance are ≥ 20 mmHg, while pediatric advanced life support changes continue to emphasize ≥ 25 mmHg for infants and ≥ 30 mmHg for children. If pressures are sub‑target despite optimal depth/rate, ensure adequate preload, minimize interruptions, and administer epinephrine per advanced cardiac life support protocols.
Embed hemodynamic cues directly into bedside algorithms:
- ETCO2 < 10 mmHg after a 2‑minute cycle: switch compressors, increase depth to 2–2.4 in (5–6 cm) in adults, recoil fully, limit ventilations, and troubleshoot airway.
- Arterial diastolic below targets: optimize chest compression fraction, confirm rate 100–120/min, ensure firm surface, give epinephrine on schedule, and consider vasopressor infusion post‑ROSC to maintain MAP.
- Abrupt ETCO2 rise: assess for ROSC at the next rhythm check and transition to post-cardiac arrest care standards (avoid hyperoxia, maintain normocapnia, treat hypotension, and implement temperature management per hospital protocol).
- Use POCUS only during planned pauses to identify reversible causes without prolonging interruptions.
Pediatrics benefit from the same physiology‑first approach, with tighter control of diastolic pressure during CPR and careful ventilation to prevent air trapping. After ROSC, target age‑appropriate blood pressures and oxygenation while avoiding hypocapnia, consistent with pediatric advanced life support changes.
Safety Training Seminars integrates these AHA 2025 guideline changes into ACLS and PALS coursework through blended learning and skills sessions that use capnography, compression feedback devices, and case‑based scenarios. With 100+ California sites, group options, and a low price guarantee, it’s a practical way to update competencies before hospital policies and audits require them.
New Standards for Neuroprognostication Following Return of Spontaneous Circulation
AHA 2025 guideline changes sharpen how and when clinicians should forecast neurologic outcome after return of spontaneous circulation. The emphasis is on delaying decisions about withdrawal of life-sustaining therapy and embedding a disciplined, multimodal approach into bedside algorithms. For California hospitals under intense throughput and legal scrutiny, the updates reduce false‑negative prognoses and support consistent documentation that aligns with post-cardiac arrest care standards.
Timing and confounder control are now front-and-center. Prognostication should be deferred until at least 72 hours after ROSC—or 72 hours after rewarming if targeted temperature management was used—and only once sedatives, paralytics, and metabolic derangements have cleared. For example, a patient on fentanyl and midazolam infusions with residual acidosis and uremia is not a candidate for reliable exam-based prognostication; bedside algorithms should force a recheck window after drug washout and correction of labs.
The guidelines reinforce a multimodal bundle, with results interpreted together rather than in isolation. Practical components include:
- Focused neurologic exam: pupillary light reflex (ideally with quantitative pupillometry), motor response, and brainstem reflexes after confounders resolve.
- Electrophysiology: continuous EEG to assess background reactivity and seizure burden; bilateral absence of N20 on SSEP as a highly specific marker when performed correctly.
- Biomarkers: serial neuron-specific enolase (NSE) trends at 24–72 hours; interpret only alongside clinical and electrophysiologic data.
- Neuroimaging: early CT for diffuse cerebral edema; MRI with diffusion-weighted imaging at 2–5 days for extent of anoxic injury.
- Structured documentation: concordance or discordance across modalities, timing relative to rewarming, and explicit listing of confounders addressed.
For pediatrics, PALS-oriented resuscitation science updates continue to caution against single-test predictions. Longer observation windows, heavy attention to sedation clearance, and family-centered communication are stressed, with EEG and MRI contributing when feasible. Pediatric advanced life support changes also encourage building age-specific decision nodes into ICU care pathways.
At the bedside, integrate these elements into advanced cardiac life support protocols and post-arrest order sets. Examples include automated EMR reminders to delay prognostication until the 72-hour mark, standardized EEG interpretation checklists, and escalation triggers for neurocritical care consults. Link debriefs on CPR performance metrics—compression fraction, rate, and end-tidal CO2—to expectations about neurologic recovery, emphasizing that better intra-arrest quality narrows prognostic uncertainty.
Safety Training Seminars helps California clinicians operationalize these updates through AHA-aligned ACLS and PALS courses that cover post-arrest pathways, EEG cases, and nuanced timing decisions. With blended learning options and 100+ locations statewide, teams can rapidly upskill and standardize neuroprognostication practices across units, while benefiting from group training and low price guarantees.
Register for a class today.